Abstract
Background: CD19-directed chimeric antigen receptor (CAR) T cell therapy is FDA approved for relapsed/refractory diffuse large B cell lymphoma (R/R DLBCL) and variants. Due to active lymphoma, "bridging" therapy may be needed to maintain disease control during the 3 or more weeks required for autologous CAR T manufacturing. Bridging therapy was not allowed in trials of axicabtagene ciloleucel (axi-cel), but could improve outcomes by reducing tumor burden. Bridging chemotherapy is one option, however toxicity and chemo-refractory disease are potential concerns. Here we report a case series of patients receiving bridging radiation therapy (XRT) prior to axi-cel therapy, of which the majority had double hit lymphoma.
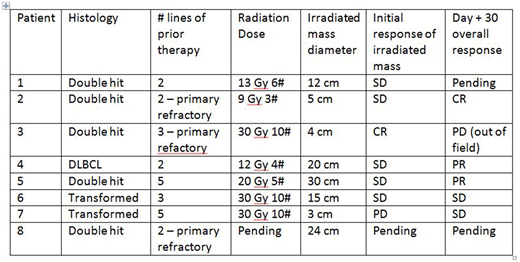
Patient Characteristics: As of July 31, 2018 eight patients received XRT as part of their bridging therapies. Overall, patients had a median of 3 prior lines of chemotherapy (range 2 to 5). Two were primary refractory to their first two lines of chemotherapy. Six of the 8 patients had double hit lymphoma by FISH. All patients received 3-4 Gy per fraction to a total median dose of 20 Gray (range 9 - 30 Gy). The most commonly used regimen was 30 Gy in 10 fractions (3/7 patients). Four patients received bridging chemotherapy in addition to XRT, mainly oral cytoxan. In 2 patients the XRT was given up until the day of leukapheresis and in 1 of these patients it was continued after leukapheresis. In 6 additional patients the XRT started after leukapheresis. Overall, XRT was completed at a median of 9 days (range 3 - 22 days) prior to starting Flu/Cy conditioning. The median mass diameter receiving XRT was 13.6 cm (range 3 - 30 cm).
Outcomes: No significant adverse events were noted at the local site of XRT before or after CAR T infusion. Response assessment within the field of radiation is complicated by a short duration between end of XRT and restaging prior to Flu/Cy conditioning. Nonetheless, only one patient experienced progressive disease in-field after XRT and most had stable disease in-field. Of the 4 cases where the baseline LDH was elevated, only the patient with PD saw their LDH rise after the completion of XRT, while the other 3 of 4 patients had declining LDH at the time of Flu/Cy conditioning.
In 7 patients evaluable for toxicity, there was one case of severe cytokine release syndrome (CRS). This event occurred in the patient who progressed through XRT and had rising LDH and declining performance status prior to CAR T infusion (this patient later died of infection). In the other 6 patients the maximum CRS was grade 1 or 2. Three out of the 7 patients had grade 3 or 4 CAR T related encephalopathy syndrome (CRES). For lymphoma outcomes at day 30, 1 patient was in CR, 2 patients PR, 2 patients with SD and 1 patient with PD (Lugano criteria). Two patients are evaluable at day 90 with PR, although one of these patients subsequently relapsed at month 4 (in-field to the prior radiation).
Conclusions: Bridging XRT (with or without chemotherapy) can be safely administered and provides disease control in refractory poor risk DLBCL patients prior to axi-cel therapy. All patients were able to receive axi-cel. CAR T-related toxicity occurred, but in this case series was most likely related to disease aggressiveness (6 of 8 patients were double hit) and tumor burden. Further research should focus on the immunologic effects of XRT and its impact on CAR T cell efficacy and toxicity.
Chavez:Humanigen: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Speakers Bureau; Merck: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Davila:Celyad: Consultancy, Membership on an entity's Board of Directors or advisory committees. Locke:Cellular BioMedicine Group Inc.: Consultancy; Kite Pharma: Other: Scientific Advisor; Novartis Pharmaceuticals: Other: Scientific Advisor.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal